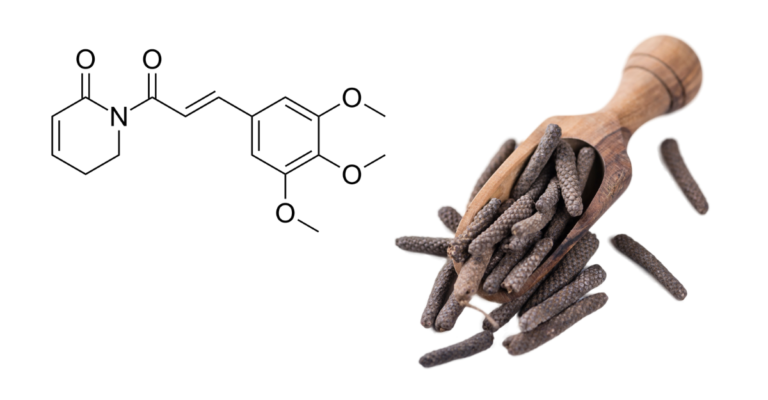
Pepper alkaloid Piperlongumine has the potential to be important in future antiviral responses because it is a host directed antiviral (HDA) that causes a stress response in infected cells rather than directly attacking the virus. Piperlongumine (PL) was found to be effective in vitro and in mouse trials against the alpha (B.1.1.7), delta (B.1.617.2), and omicron (B.1.1.529) forms of SARS-Cov-2.
“This is quite advantageous because PL acts on the cells infected by the virus rather than the virus itself,” said. (This) makes it a potential antiviral treatment that could be effective in future variants of SARS-CoV-2 or even different viruses to combat future outbreaks.
Gonçalo Bernardes, a researcher at Lisbon’s Instituto de Medicina Molecular and Cambridge University.
PL is a long pepper plant alkaloid/imide extract that has been used in Ayurvedic medicine. It was also used in cancer therapy trials, where researchers discovered that it worked by inducing a stress response in host cells.
In their study, published in ACS Central Science, the Bernardes group stated that HDAs were “believed to be more effective against SARS-CoV-2 variants of concern (than direct-acting antivirals, or DAAs) because host genes have a low tendency to change.” Because DAAs such as remdesivir, paxlovid, and molnupiravir have been the majority of antiviral drugs discovered thus far, a new study to assess the true efficacy of HDAs appears especially important.
How Pepper alkaloid Piperlongumine works as a anti-SARS-CoV-2
Researchers discovered that “PL demonstrates significant anti-SARS-CoV-2 action, with low micromolar potency” after testing it on two cell lines, VERO-CCL 81 and A549-hACE2, and that it could be used in both preventative and therapeutic situations. They then used a transgenic mouse model of SARS-Cov-2 infection (K18-hACE2 mice) to demonstrate that PL had “robust” antiviral efficacy in vivo against the various variants. PL also outperformed plitidepsin, a promising antiviral that is currently being tested in humans and was used as a control in the Bernardes study.
Another encouraging finding from the group’s in vivo mouse studies was that PL is especially well-suited to nasal administration – a potentially effective treatment route “because the nasal mucosa is frequently the primary site of infection in SARS-CoV-2.” The researchers were also able to demonstrate that intranasal administration of PL at 1 mg/kg was not toxic to mice, and they speculated that PL could eventually become a self-administered nasal drug for humans, “which would decrease the requirement for professional healthcare labor and increase(e) patient compliance.”
The group also discussed how their research focused on PL’s role in the selective induction of Reactive Oxygen Species (ROS) in infected cells, writing: “Given that PL selectively kills cancer cells but not normal cells by inducing ROS, we investigated if PL could also selectively induce ROS in SARS-CoV-2 infected cells.”
“The selective accumulation of ROS in infected cells is shown to direct the antiviral effect of PL,” they added.
“PL increases ROS levels and decreases reduced glutathione by inhibiting the upregulated pi-class glutathione S-transferase (GSTP1) in infected cells” (GSH). Following ROS-mediated activation of mitochondrial antiviral-signaling protein (MAVS), the IFN-JAK-STAT pathway is activated, leading to viral degradation.
“As an HDA, PL does not directly target the virus but rather selectively affects the ROS level in infected cells, implying that PL has the potential to become a pan-SARS-CoV-2 therapeutic and would be useful against emerging SARS-CoV-2 VOCs (variants of concern).”
CWS Abroad is a TRC distributor and supplies the reference standard Piperlongumine CAS Number: 20069-09-4 throughout Brazil and Latin America.