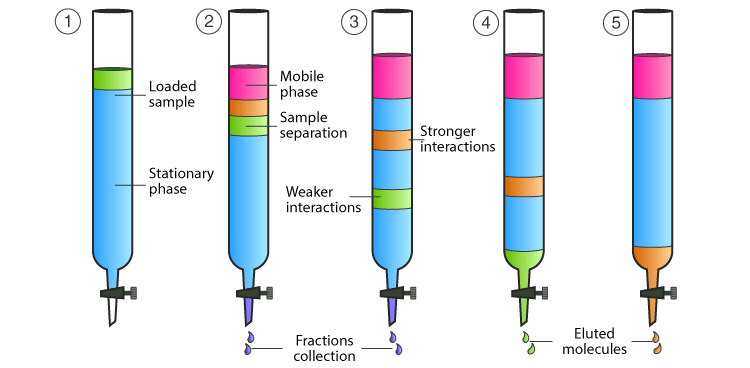
In chemistry, Column chromatography is a technique which is used to separate a single chemical compound from a mixture dissolved in a fluid. Column chromatography separates substances based on differential adsorption of compounds to the adsorbent as the compounds move through the column at different rates which allows them to get separated in fractions.
This technique can be used on a small scale as well as large scale to purify materials that can be used in future experiments. This method is a type of adsorption chromatography technique.
Column Chromatography Principle
When the mobile phase along with the mixture that needs to be separated is introduced from the top of the column, the movement of the individual components of the mixture is at different rates. The components with lower adsorption and affinity to the stationary phase travel faster when compared to the greater adsorption and affinity with the stationary phase. The components that move fast are removed first whereas the components that move slowly are eluted out last.
The adsorption of solute molecules to the column occurs in a reversible manner. The rate of the movement of the components is expressed as:
Rf = the distance travelled by solute/ the distance travelled by the solvent
Rf is the retardation factor.
Elution
Elution is a chemical process that includes exchanging ions with another substance to remove the ions from one material. A solvent-based chromatographic method for extracting an adsorbed material from a solid adsorbing medium. The solvent or mobile phase that passes through the column is referred to as the eluent. The molecules desorb from the adsorbent and dissolve in the eluent when the polarity of the eluent matches the polarity of the molecules in the sample.
Eluent is the proportion of the mobile phase that carries the sample components. An eluate is the combination of solute and solvent that leaves the column. The mobile phase and analytes are combined to form the eluate. A material that separates and transports elements of a mixture through a chromatograph column. In liquid chromatography, the eluent is a liquid solvent, but in gas chromatography, it is a carrier gas.
Procedure for Column Chromatography
Before we begin the Column Chromatography Experiment, let us first grasp the various stages involved.
Mobile phase – This phase is composed of solvents and serves the following purposes:
- It functions as a solvent-sample mixture that may be added to the column.
- It functions as a developing agent, assisting in the separation of constituents in the sample to create bands.
- It serves as an eluting agent, removing the components separated during the experiment from the column.
- Solvents used as mobile phases based on their polarity include ethanol, acetone, water, acetic acid, pyridine, and others.
Stationary phase – A solid substance with good adsorption capabilities that meets the following conditions:
- Particle shape and size: Particles should be consistent in shape and size, ranging from 60 to 200 microns in diameter.
- Particle stability and inertness: strong mechanical stability and chemical inertness. In addition, no acids, bases, or other solvents were employed during the experiment.
- It should be colorless, cheap, and widely available.
- Mobile phase should be free to flow.
- It should be suited for the separation of diverse chemical combinations.
Experiment with Column Chromatography
- As the upper level of the mobile phase and the stationary phase should coincide, the stationary phase is made moist with solvent. The mobile phase or eluent might be a solvent or a solvent combination. The compound mixture that has to be separated is introduced from the top of the column without disrupting the top level in the first stage. The water is switched on, and the adsorption process on the silica surface begins.
- The solvent combination is progressively introduced to the glass column without disturbing the stationary phase. The solvent is introduced as needed throughout the experiment.
To start the flow of chemicals in the mixture, the tap is turned on. The polarity of the molecules in the sample determines the movement. When compared to the polar components, the non-polar components move faster. - If a chemical mixture has three distinct compounds, say red, blue, and green, its polarity order will be blue>red>green.
- Because the green compound has a lower polarity, it will migrate first. It is collected in a clean test tube when it reaches the end of the column. Following that, the red compound is collected, followed by the blue compound. All of these are gathered in different test tubes.
Applications of Column Chromatography
- To isolate active compounds, column chromatography is utilized.
- It is quite useful for separating chemical mixtures.
- It is used to estimate drug concentrations from medication formulations.
- Its purpose is to eliminate contaminants.
- Metabolites are isolated from biological fluids using this method.
Frequently Asked Questions
What is the principle involved in column chromatography?
The basic idea behind column chromatography is to adsorb solutes from a solution using a stationary phase and then separate the mixture into distinct components.
What is column chromatography?
It is a preparatory strategy for purifying chemicals based on their hydrophobicity or polarity. The molecular mixture is separated in this chromatography procedure based on its differential partitioning between a stationary phase and a mobile phase.
What is the main advantage of column chromatography?
The key benefit of this chromatography approach is that the stationary phase is less expensive and can be readily disposed of because it is recycled.
How are the compounds separated in this technique?
The separation is similar to TLC in that the chemical mixture is transported by a mobile phase through a stationary phase.
Which compounds elute out first in the column chromatography technique?
Compounds that are not polar. When contrasted to non-polar molecules, polar compounds will significantly commune with silica.
What is elution in column chromatography?
Elution is a chromatographic method that uses a solvent to remove an adsorbed material from a solid adsorbing substrate.
What are the limitations of column chromatography?
Column chromatography is a method of separating a single chemical ingredient from a solution mixture. Column chromatography has a few limitations. It is substandard, difficult, expensive, and time-consuming.
What are the different types of column chromatography?
Adsorption column chromatography, partition column chromatography, gel column chromatography, and ion-exchange column chromatography are the four types of column chromatography.
What is an adsorption column chromatography?
Adsorption column chromatography is a type of column chromatography in which the mixture’s components are adsorbed on the adsorbent’s surface.
What is gel column chromatography?
Gel column chromatography is a kind of column chromatography in which the separation takes place in a gel-packed column. The immobile phase in gel column chromatography is a solvent retained in a solvent gap.
With more than 20 years of experience on the market, CWS Abroad is a firm with a wealth of knowledge in the customs services, sales of products and equipment for analytical and research laboratories, chemical products, and consumables.
Follow us on LinkedIn and Instagram to stay up to date on life science industry news and learn more about our work!

